Addressing the Anatomic Substrate of OSA
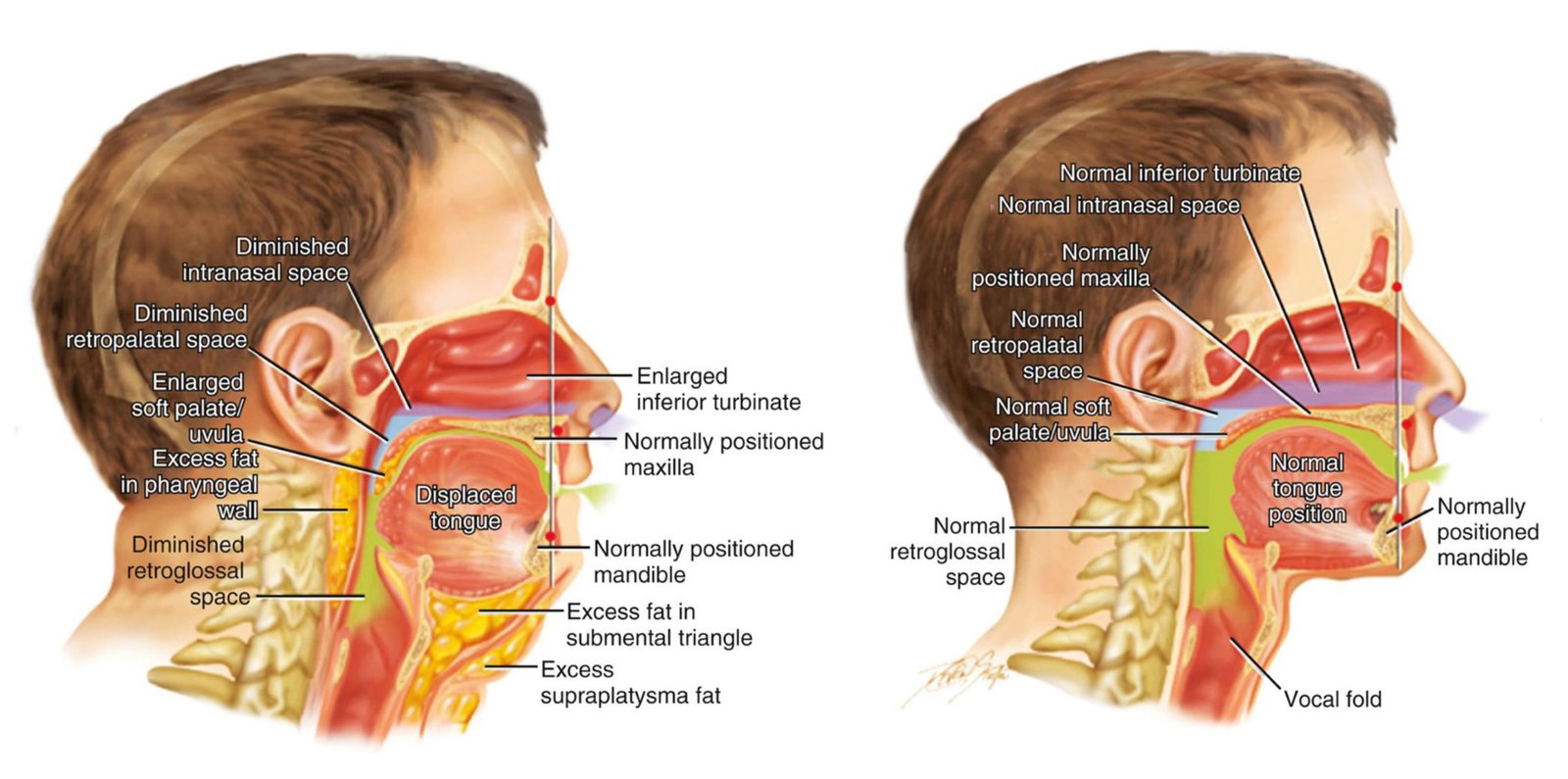
Recent MRI volumetric studies have established that tongue and parapharyngeal fat deposition represents a primary pathophysiologic mechanism in obstructive sleep apnea, independent of overall BMI.
- Wang et al., AJRCCM (2020): "Effect of Weight Loss on Upper Airway Anatomy and the Apnea-Hypopnea Index: The Importance of Tongue Fat" — MRI volumetric analysis demonstrates 32% weight loss produces 48% reduction in tongue fat (14,126mm³ to 7,337mm³) with corresponding 80% decrease in AHI (121 to 24.6)
- Parapharyngeal and retroglossal fat deposits shown to narrow pharyngeal airway diameter independent of BMI and total neck circumference
- Bariatric surgery outcomes confirm AHI improvement occurs disproportionately early relative to total body weight reduction
- Localized adipose reduction in upper airway structures represents mechanistic target independent of systemic weight loss
- Clinical Trial NCT06949969: Randomized IRB study demonstrating 100% efficacy in submental fat reduction and 80% improvement rate in sleep apnea severity